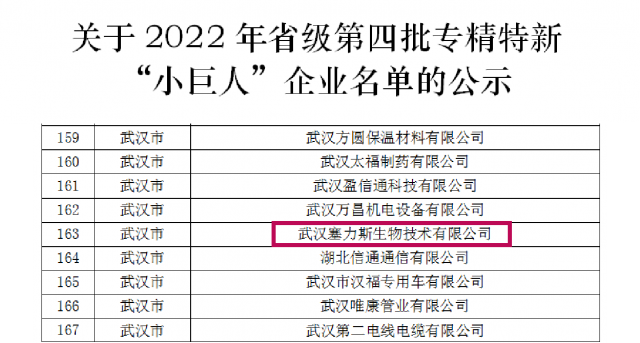
Recently, ThalysBio has been successfully recognized as a provincial specialized, special and new “small giant” enterprise.
To be recognized as a specialized, precise, characteristic and innovative “Little Giant” enterprise, one needs to meet six indicators: specialization (focus / deep cultivation), precision (specification / system), characterization (unique / competitive), innovation (innovation / independence), chain (industrial chain / application), product (core / manufacturing) six indicators, boasting core traits of specialization, precision, characterization and innovation in its main business field. ThalysBio’s recognition as “Little Giant” enterprise is the result of the comprehensive evaluation of company’s focus on the IVD (blood coagulation) professional market segment for many years and our ability in research and innovation, high market share, mastery of key core technologies, and excellent quality and efficiency.
Specialization
ThalysBio, as an enterprise deeply engaged in thrombosis and hemostasis diagnosis for decades, has established two technical platforms of clinical in vitro diagnostic reagents and clinical testing and analysis instruments, and has gradually realized the goal of “serialization”, “integration” and “localization” of reagents and instruments, covering the R&D and intellectual manufacturing system of coagulation diagnosis, chemiluminescence, POCT (point-of-care testing), autoimmune and microbiological testing.
Precision
ThalysBio boasts a world-class production environment and production management process, and has passed the dual international quality system certification of IS013485 and IS09001 as well as CE product certification. The reagents and instruments embrace perfect production processes and reliable quality, and its coagulation products are the only domestic products that has been certified by the British RANDOX third-party quality control system so far, providing a strong guarantee for accurate clinical results.
Characterization
ThalysBio, as the manufacturer with the most complete thrombosis and hemostasis product line (instruments, reagents and consumables) in China, has been approved to register as many as 56 items of coagulation diagnostic products, covering a wide range of routine testing, special testing, drug testing and other projects. With the core technology of its testing instruments, ThalysBio is able to detect abnormal plasma and monitor coagulation curves in real time with excellent anti-interference ability.
Innovation
ThalysBio conducts innovative R&D based on clinical needs. In addition, it also actively harnesses external development forces to promote the common progress of technology and clinical medicine through the innovation mode of “industry-education-research-medicine”. It has also established long-term partnerships with well-known domestic academic research institutions such as Huazhong University of Science and Technology, Shanghai Jiao Tong University and Cyrus Tang Hematonosis Research Center of Jiangsu University to further enhance its R&D strength.
1 专业化
塞力斯生物作为深耕血栓与止血诊断领域数十年的企业,创建了临床体外诊断试剂和临床检测分析仪器两大技术平台,逐步实现了试剂和仪器“系列化”、“一体化”、“国产化”的目标,覆盖了凝血诊断、化学发光、POCT、自身免疫、微生物检测等方向的研发智造体系。
2 精细化
塞力斯生物建立了国际一流的产品生产环境及生产管理流程,通过了ISO13485、ISO9001双重国际质量体系认证以及CE产品认证。试剂和仪器生产工艺完善,质量可靠,其凝血产品是迄今为止国内唯一获得英国RANDOX第三方质量控制体系认证的国产产品,为提供准确的临床结果提供了有力的保障。
3 特色化
作为国内目前拥有最完整的血栓与止血产品线(仪器、试剂、耗材)的厂家,塞力斯生物凝血诊断产品获批注册许可的项目多达56项,广泛覆盖常规检测、特殊检测及药物检测等项目。塞力斯生物凭借其检测仪器的核心技术,能实现对异常血浆的检测,可实时监测凝固曲线,抗干扰能力出色。
4 新颖化
塞力斯生物以临床需求为导向进行创新研发,除依靠自身研发能力外,积极借助外部开发力量,通过“产学研医”相结合的创新模式,推动技术与临床医学的共同进步。与华中科技大学、上海交通大学、江苏大学唐仲英血液病研究中心等国内知名学术研究机构建立了长期的合作关系,进一步提升研发实力。